Introduction:Daratumumab, a human IgGκ monoclonal antibody targeting CD38, has shown clinical efficacy in combination with standard-of-care regimens in patients with newly diagnosed multiple myeloma (NDMM). The addition of daratumumab to bortezomib, melphalan, and prednisone (D-VMP) significantly improved clinical outcomes vs bortezomib, melphalan, and prednisone (VMP) in phase 3 studies in Asian patients (OCTANS) and a global population (ALCYONE) with transplant-ineligible (TIE) NDMM. Here, we present subgroup analyses of progression-free survival (PFS) by measurable residual disease (MRD) and cytogenetic risk status from OCTANS and ALCYONE.
Methods: Eligible patients from OCTANS (NCT03217812) and ALCYONE (NCT02195479) had a diagnosis of NDMM and were TIE due to age or comorbidities. Patients were randomized to receive up to nine 42-day cycles of VMP (bortezomib 1.3 mg/m 2 SC twice weekly in Cycle 1 and weekly in Cycles 2-9, and melphalan 9 mg/m 2 and prednisone 60 mg/m 2 orally on Days 1-4 of each cycle) or D-VMP (VMP + daratumumab 16 mg/kg IV weekly in Cycle 1, every 3 wk in Cycles 2-9, and every 4 wk thereafter until disease progression or unacceptable toxicity). MRD negativity (10 -5) was assessed by multi-parameter flow cytometry in OCTANS and next-generation sequencing in ALCYONE. Cytogenetic risk was assessed by FISH or karyotype testing.
Results:A total of 220 Asian patients were randomized (D-VMP, n = 146; VMP, n = 74) in OCTANS, and 706 global patients were randomized (D-VMP, n = 350; VMP, n = 356) in ALCYONE. With a median follow-up of 41.2 mo (range, 0.0-59.9) in OCTANS, the median PFS was 38.7 mo with D-VMP and 19.2 mo with VMP (HR, 0.35; 95% CI, 0.23-0.52; P <0.0001). With a median follow-up of 40.1 mo (range, 0.0-52.1) in ALCYONE, the median PFS was 36.4 mo with D-VMP and 19.3 mo with VMP (HR, 0.42; 95% CI, 0.34-0.51; P <0.0001).
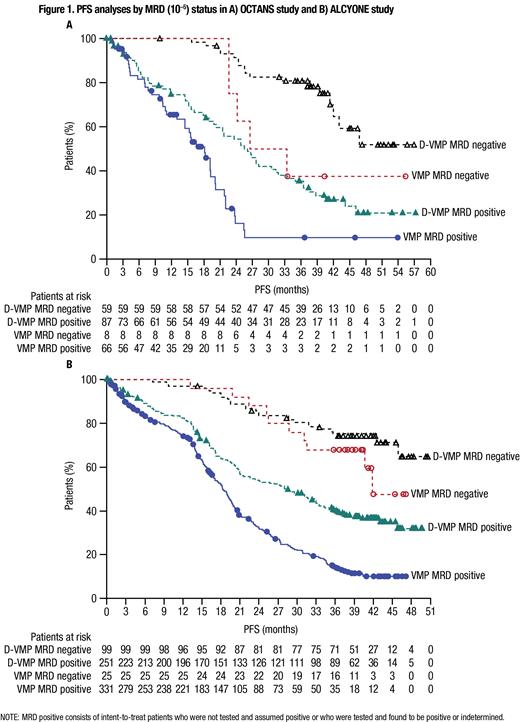
The rates of overall and sustained MRD negativity were higher with D-VMP vs VMP. In OCTANS, the MRD-negativity rate was 40.4% with D-VMP vs 10.8% with VMP ( P <0.0001), and more patients in the D-VMP group had sustained MRD negativity lasting ≥12 mo (24.7% vs 1.4%; P <0.0001) and ≥18 mo (15.1% vs 1.4%; P = 0.0008). Similarly, in ALCYONE, the MRD-negativity rate was 28.3% with D-VMP vs 7.0% with VMP ( P <0.0001), with more patients in the D-VMP group having sustained MRD negativity lasting ≥12 mo (14.0% vs 2.8%; P <0.0001) and ≥18 mo (8.9% vs 1.7%: P <0.0001). In both studies, longer PFS was observed for patients who achieved MRD negativity vs those who did not within each treatment group; however, the PFS benefit observed for D-VMP vs VMP was maintained irrespective of MRD status ( Figure). In multivariate analyses, achievement of sustained MRD negativity lasting ≥12 mo was associated with longer PFS vs MRD negativity <12 mo in both OCTANS (HR, 0.23; 95% CI, 0.09-0.62; P = 0.004) and ALCYONE (HR, 0.13; 95% CI, 0.06-0.32; P <0.0001). No other factors showed a significant association with PFS in the multivariate analysis for OCTANS; other factors associated with longer PFS in ALCYONE included age <75 vs ≥75 y (HR, 0.38; 95% CI, 0.20-0.75; P = 0.005) and ISS disease stage I vs II (HR, 0.20; 95% CI, 0.05-0.86; P = 0.03).
Data from OCTANS and ALCYONE were pooled for an analysis of PFS by cytogenetic risk subgroup (D-VMP, n = 496; VMP, n = 430). D-VMP prolonged median PFS vs VMP in patients with standard cytogenetic risk (not estimable vs 18.9 mo; HR, 0.33; 95% CI, 0.26-0.42) and high cytogenetic risk (25.6 vs 18.9 mo; HR, 0.54; 95% CI, 0.41-0.71), defined by the presence of t(4;14), t(14;16), del(17p), t(14;20), gain(1q21), and/or amp(1q21). Median PFS was also longer with D-VMP vs VMP for patients with gain and/or amp(1q21) (31.9 vs 18.9 mo; HR, 0.45; 95% CI, 0.32-0.63), 1 high-risk cytogenetic abnormality (HRCA; 24.4 vs 19.3 mo; HR, 0.62; 95% CI, 0.44-0.86), and ≥2 HRCA (28.2 vs 17.5 mo; HR, 0.36; 95% CI, 0.20-0.63).
Conclusions:In this subgroup analysis of OCTANS and ALCYONE, the prolonged PFS observed with D-VMP vs VMP in the overall study populations was maintained irrespective of the achievement of MRD negativity or the presence of high cytogenetic risk. In addition, the rates of MRD negativity and sustained MRD negativity were higher with D-VMP vs VMP, and achievement of these deep responses translated into longer PFS, consistent with a prior pooled analysis of the MAIA and ALCYONE studies. These results further support the use of daratumumab in combination with VMP in both Asian and global patients with TIE NDMM.
Disclosures
Huang:Janssen Pharmaceutical Ltd: Speakers Bureau. Rodriguez Otero:GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees, Other: Honoraria for lectures; Bristol Myers Squibb: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Honoraria for lectures; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Honoraria for lectures; Sanofi: Membership on an entity's Board of Directors or advisory committees, Other: Honoraria for lectures; Amgen: Other: Honoraria for lectures; Regeneron: Other: Honoraria for lectures; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Oncopeptides: Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel grants; Roche: Consultancy. Dimopoulos:GlaxoSmithKline: Honoraria, Membership on an entity's Board of Directors or advisory committees; Regeneron: Honoraria, Membership on an entity's Board of Directors or advisory committees; Menarini: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; BeiGene Inc: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; AbbVie: Honoraria, Membership on an entity's Board of Directors or advisory committees. Wroblewski:Janssen: Current Employment, Current equity holder in publicly-traded company. Borgsten:Janssen: Current Employment, Current holder of stock options in a privately-held company. Carson:Janssen Research & Development, LLC: Current Employment. Liu:Johnson & Johnson China Ltd: Current Employment, Current holder of stock options in a privately-held company. Chen:Xian Janssen Pharmaceutical Ltd.: Current Employment, Current holder of stock options in a privately-held company. Cui:Xian Janssen Pharmaceutical Ltd.: Current Employment, Current holder of stock options in a privately-held company.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal